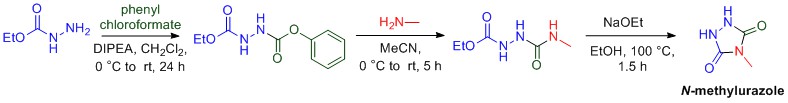
Amine (nitrogen) containing compounds are especially prevalent in pharmaceuticals, with over 70% of marketed pharmaceuticals containing the amine functional group. In addition to amines, chiral compounds are also prevalent in pharmaceuticals. The principle of chirality is most easily understood when considering your hands. Your right and left hands are mirror images of each other, but are non-superimposable. This principle of being non-superimposable is what prevents your right hand from fitting in a left-handed glove, despite the fact that your right and left hand both have four fingers and a thumb.
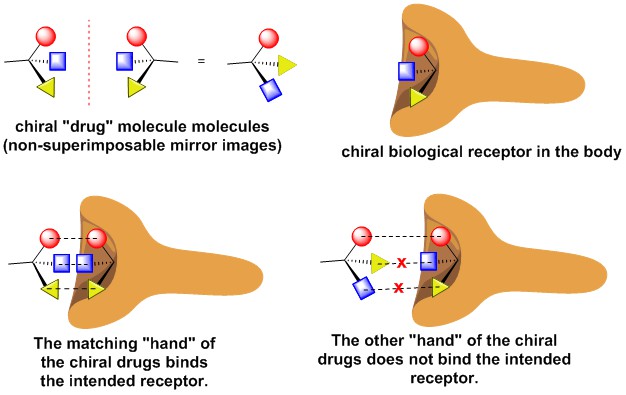
Molecules can also be chiral and the principle of chirality has huge implications in medicine. Often times only one the right or left-hand of the chiral molecule has the intended biological consequence in the body, while the other hand of the molecule can many times have a harmful effect. It is important for organic chemists to develop methods to prepare chiral amines in which only one “hand” of the molecule is prepared. Organic methodology in our lab is focused on the preparation of chiral amine containing compounds, as these types of molecules are important molecular structures in many pharmaceutical compounds.
Completed Projects:
Stereoselective Synthesis of 2-Substituted 1,2,5,6-Tetrahydropyridines and Piperidines: Chiral 2-substituted piperidines (6-membered rings containing nitrogen) are found in many drug and biologically active molecules. Due to their prevalence, many methods for the synthesis of these compounds has been developed. However, very few synthetic methods have been developed for the synthesis of 2-substituted 1,2,5,6-tetrahydropyridines (piperidines containing a double bond within the piperidine ring). The presence of the double bond is advantageous as it provides a functional handle for the synthesis of more complex piperidine ring systems. From 2015-2016 research students Danielle Penk, Nathan Robinson, and Harrison Hill designed an efficient method for the stereoselective synthesis of 2-substituted 1,2,5,6-tetrahydropyridines and piperidines.
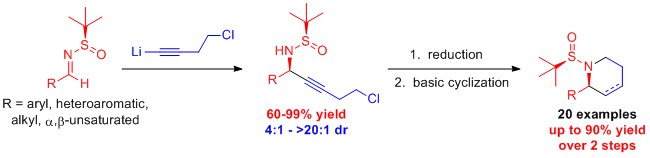
Synthesis of Chiral Terminal Halo-Substituted Propargylamines: Propargylamines are compounds that containing an amine (a nitrogen atom) adjacent to an alkyne (a triple bond). These molecules are useful synthetic intermediates for more highly functionalized molecules. Adding a halogen (i.e. chlorine or bromine) to the alkyne further increases the synthetic flexibility of this class of molecules. From 2014-2015 research students, Savannah Jordan, Samuel Starks, and Michael Whatley designed a method to prepare chiral chloro-substituted propargylic amines with high diastereoselectivity and yield. The reactivity of these compounds to prepare further functionalized compounds was also demonstrated. Currently the Turlington lab is continuing to extend the usefulness of this methodology by developing reaction conditions to prepare chiral bromo-substituted propargylic amines.
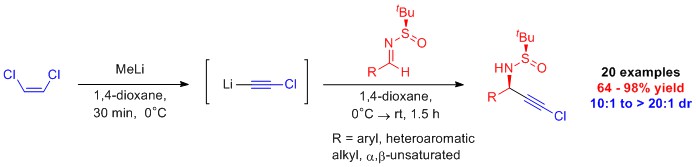
Synthesis of N-Alkyl Urazoles: Urazoles are precursors to 1,2,4-triazoline-3,5,-diones (TADS) that are used in a variety of contexts, among these medicinal chemistry. Until very recently N-methylurazole was commercially available. The high toxicity of methyl isocyanate, the conventional precursor N-methylurazole, has made large scale commercial and academic synthesis of N-methylurazole unfeasible, and is likely the cause for N-methylurazole no longer being commercially available. Collaborative work between our lab and Dr. Gary Breton’s lab resulted in the development of methods for the synthesis of gram quantities of N-methylurazole and N-alkylurazole derivatives.